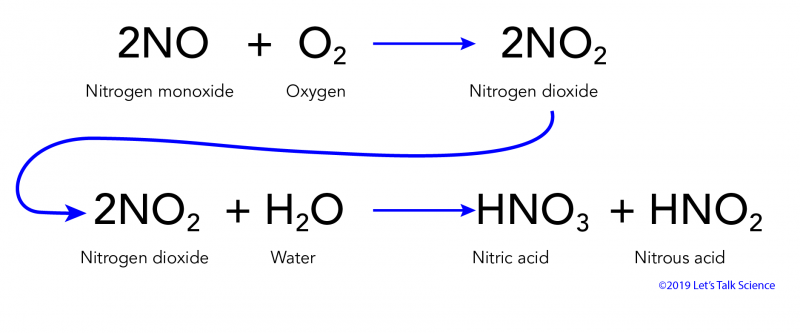
Nitrogen Dioxide Reacts With Water to Form Nitric Acid
1 mol NO2 3 mol NO2 1 mol H2 O 3 mol H2O. Compound amount NO2 75 g H0 1699 g HNO3.
What Is Acid Rain Let S Talk Science
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation.
. 3 NO2 g H2O l -- 2HNO3 l NO g Suppose that 5 mol NO2 and 1 mol H2O combine and react completely. Nitric oxide combines rapidly with oxygen in the air to form nitrogen dioxide. The nitrate radical subsequently reacts with aldehydes RCHO or water to form nitric acid.
How many moles of the reactant in excess are present after the reaction has completed. Nitrogen dioxide and water react to form nitric acid and nitrogen monoxide like this. The first thing to do here is identify your reactants and your product.
3NO2gH2Olâ 2HNO3lNOg Suppose that 43 mol NO2 and 080 mol H2O combine and react completely. This reaction is a. Nitrogen dioxide reacts with water to form nitric acid and nitric oxide reacts with alkalis to form nitrates and nitrites Merck 11th ed.
Nitrogen dioxide gas reacts with water to produce nitric acid and nitrogen monoxide. Which reactant is in excess. 4NO2g O2g 2H2Ol gt.
2 mol NO2g 1 mol H2O1 3 mol NO2 mot HEOL. Nitric acid is a component of acid rain that forms when gaseous nitrogen dioxide pollutant reacts with gaseous oxygen and liquid water to form aqueous nitric acid. Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation.
Nitrogen Dioxide NO2 reacts with water H2O to form Nitric acid HNO3 and Nitrogen Monoxide NO. Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation. Upon dissolving the nitrogen dioxide reacts with water to form nitric acid as indicated by the color change of the solution.
Nitrogen dioxide is also produced in the reaction. NO 2 then reacts with OH to form nitric acid HNO 3 which contains nitrogen in the V oxidation state. As more brown nitrogen dioxide forms more of.
If 5 moles NO2 and 1 mol H0 combine and react completely. How many moles of the reactant in excess are present after the reaction has completed. The resulting pressure difference draws more oxygen into the flask when the stopper is loosened forming more NO 2.
The balanced chemical equation of the reaction is 3 NO2 g H2O l 2HNO3l NO g Here in this problem given atfirst you have to determin. This reaction happens very easily in water. The problem tells you that gaseous nitrogen dioxide NO2 reacts with gaseous oxygen O2 and liquid water H2O to form aqueous nitric acid HNO3.
3NO2g H2Ol - 2HNO31 NOg. 3 NO2g H2Ol HNO3l NOg Suppose that 4mol NO2 and 2 H2O mol combine and react completely. Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation.
How many moles of. Chlorobenzene C6H5Cl is used in the production of chemicals such as aspirin and dyes. The nitrogen dioxide dissolves in the solution creating a partial vacuum within the flask.
Brown color of nitrogen dioxide gas is disappeared when reaction occurs. Therefore produced nitrogen dioxide gas is sent to water to manufacture nitric acid in chemical industry. Nitric acid also can be formed at night through reactions involving the nitrate radical NO 3 a product of the reaction of NO 2 and O 3.
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation. 3 NO 9 HO1 2 HNO 1 NO9 Suppose that 14 mol NO and 4 mol H30 combine and react completely How many moles of the roactant in. 3 NO2 gH2 O l 2 HNO3 NO g Suppose that 12 mol NO2 and 3 mol H2 O combine and react completely.
NO2g the g is used to denote a compound in the gaseous form. If 5 moles NO2 and 1 mol H2O combine and react completely. How many moles of the reactant in excess are present after the reaction has completed.
Nitrogen dioxide NO2 reacts with ____ in the atmosphere to form nitric acid HNO3 asked Jun 25 2017 in Environmental Atmospheric Sciences by Keyboard. Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation. Express your answer as a chemical formula.
The liquid nitrogen oxide is very sensitive to detonation in the presence of water. Nitrogen dioxide NO2 is an acidic gas and readily reacts with water H2O to produce an acidic solution of nitric acid HNO3 and nitrogen monoxide NO. Ozone O3 in the atmosphere can be converted to oxygen gas by reaction with nitric oxide NO.
3 NOgH012 HNO3aqNOg At a certain temperature a chemist finds that a 75 L reaction vessel containing a mixture of nitrogen dioxide water nitric acid and nitrogen monoxide at equilibrium has the following composition. Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation. 3 NO2 g H2O l -- 2HNO3 l NO g Suppose that 5 mol NO2 and 1 mol H2O combine and react completely.
3NO2 g H2O l - 2HNO30 NO g. Up to 256 cash back Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation. How many moles of the reactant in excess.

How To Balance No2 H2o Hno3 No Youtube
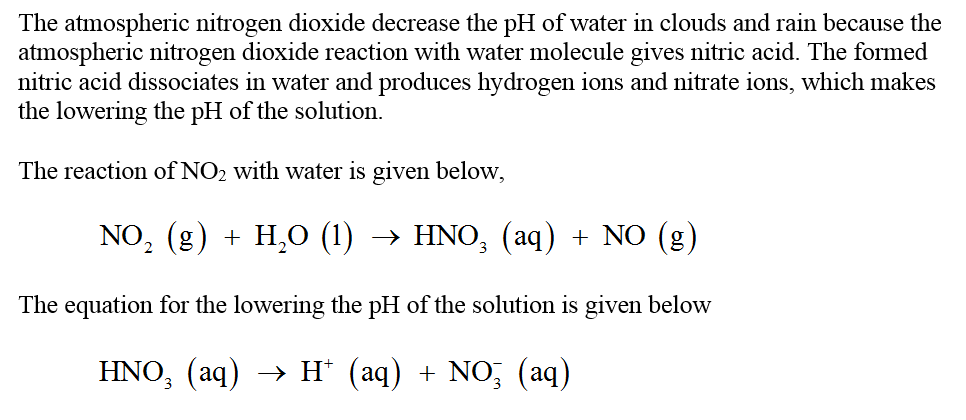
Answered Explain How Atmospheric Nitrogen Bartleby

How To Balance Hno3 H2o No2 O2 Nitric Acid Decomposing Youtube
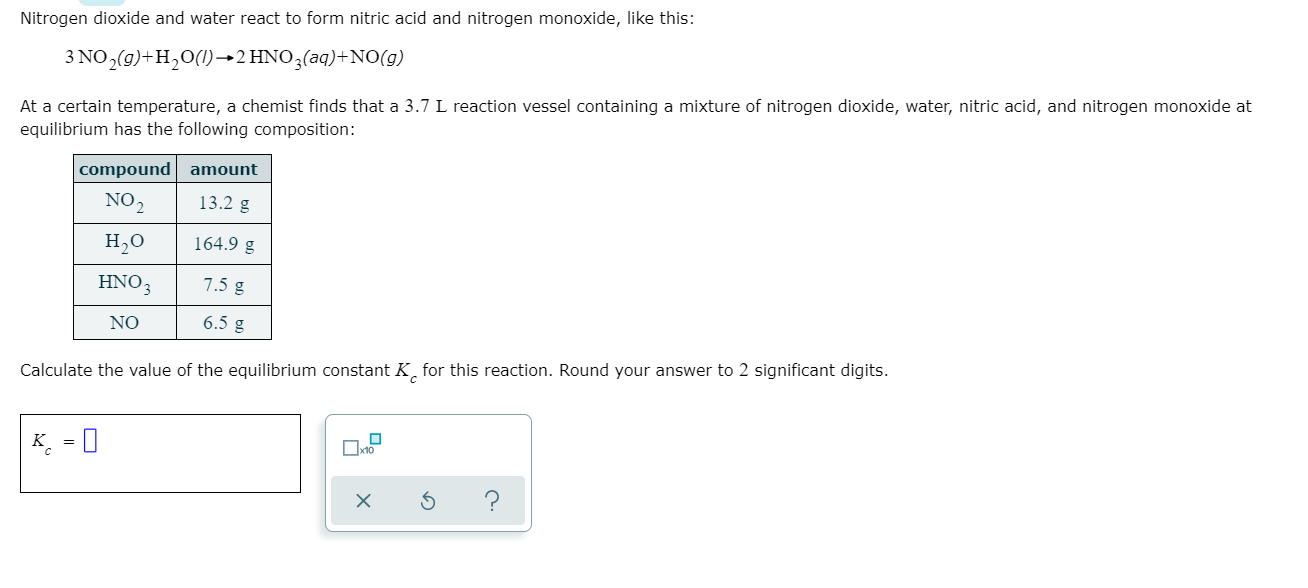
Solved Nitrogen Dioxide And Water React To Form Nitric Acid Chegg Com
Nitrogen Oxides In The Atmosphere
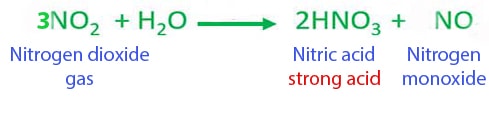
Nitrogen Dioxide And Water Reaction No2 H2o

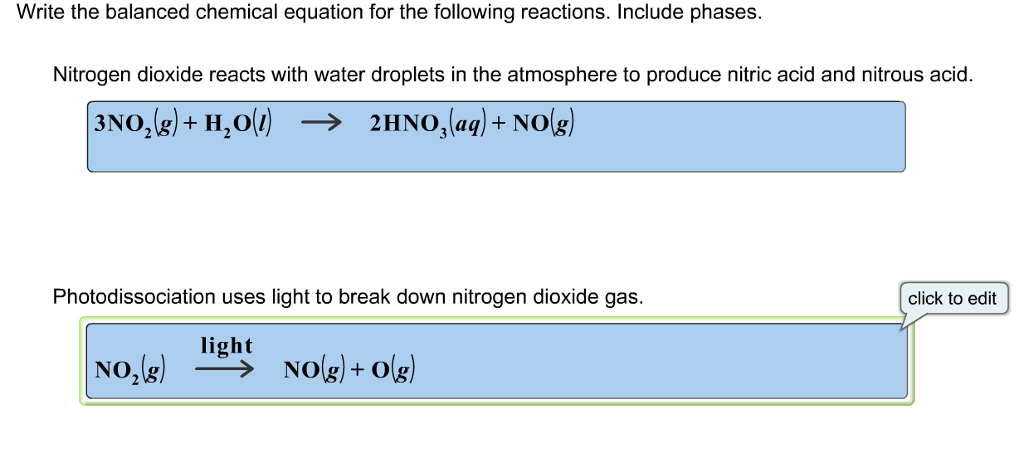
Oneclass Write The Balanced Chemical Equation For The Following Reactions Include Phases Nitrogen D
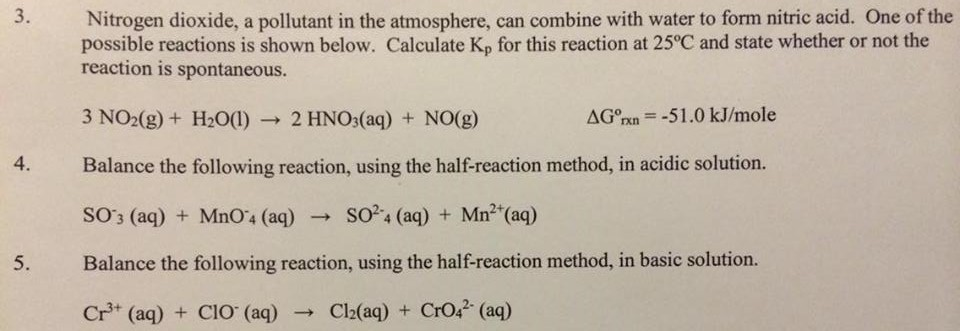
Solved Nitrogen Dioxide A Pollutant In The Atmosphere Can Chegg Com

How To Balance No2 H2o Hno2 Hno3 Nitrogen Dioxide Water Youtube
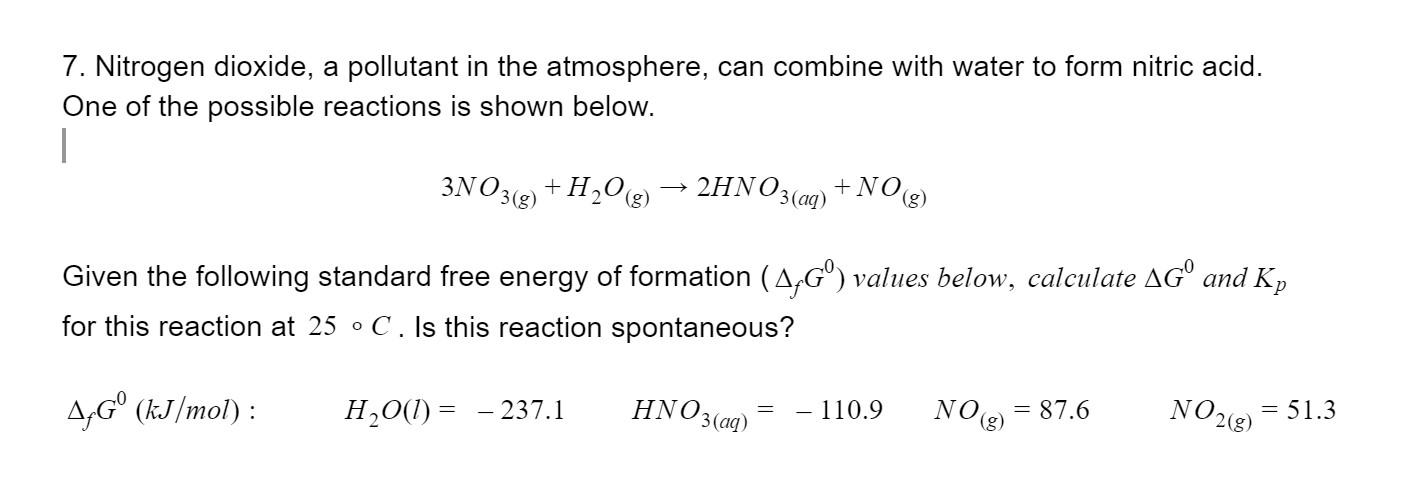
Solved 7 Nitrogen Dioxide A Pollutant In The Atmosphere Chegg Com

Solved 4 Nitric Acid And Nitrogen Monoxide Are Produced When Chegg Com

Human Activity Adversely Affect Human Activity Ozone Depletion How To Level Ground
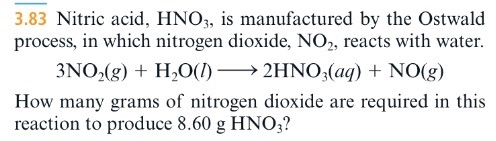
Solved 3 83 Nitric Acid Hno3 Is Manufactured By The Chegg Com

Oneclass Write The Balanced Chemical Equation For The Following Reactions Include Phases Nitrogen

Solved Write The Balanced Chemical Equation For The Chegg Com

Solved Nitrogen Dioxide No2 Gas And Liquid Water H2o Chegg Com

Nitrogen Dioxide An Overview Sciencedirect Topics

Is No2 Nitronium Ion Polar Or Nonpolar Nitrogen Dioxide Polar Molecules

Comments
Post a Comment